In 2018, EATRIS-ERIC, GlaxoSmithKline (GSK) and five EATRIS member institutions (Academic Medical Center Amsterdam, Radboud University Medical Center Nijmegen, University Medical Center Groningen, VU University Medical Center Amsterdam from the Netherlands, and Uppsala University and Uppsala University Hospital from Sweden) announced the establishment of a novel international public-private partnership: The Translational Molecular Imaging Hub.
The Hub delivers a clinical and scientific expert network for the development and application of innovative imaging methods for inflammatory diseases. Applying imaging in information-rich, small cohort studies can provide a high, immediate impact to enhance the productivity of research and development: developing our understanding of disease in the patient; enriching clinical trial cohorts; measuring therapeutic response.
Now at the end of the initial collaboration period, EATRIS-ERIC, EATRIS member institutions and GSK, have expressed their commitment to continue their partnership for a minimum of three more years. Already, the Imaging Hub has built a mature project portfolio comprising of three preclinical projects and two clinical projects, which hold great translational potential. Partners are also involved in a joint PhD programme supported by the Marie Skłodowska Curie programme of Horizon 2020.
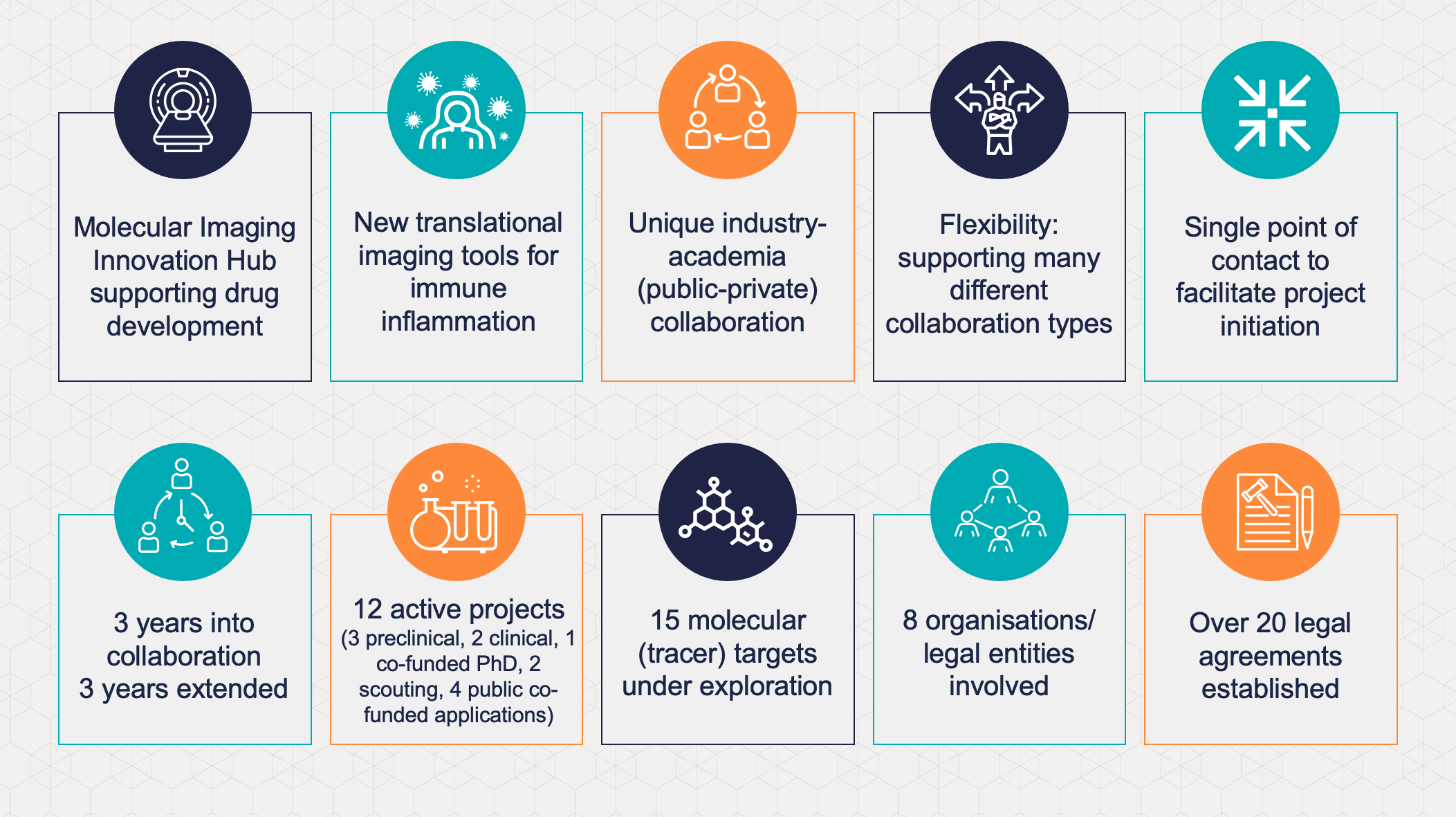
A growing project portfolio supported by a flexible legal framework
The flexibility of the Innovation Hub legal framework allows it to support several project types with pre-set terms (e.g. Intellectual Property, publication), with EATRIS-ERIC acting as the Hub Secretariat and portfolio manager, playing a key role in administering the Hub’s operations. Over 20 legal agreements have been prepared to date (including multilateral Confidentiality Disclosure Agreements, Material Transfer Agreements, third party licensing, project agreements, external consortium agreements) and proposals can be individually tailored to support the collaboration. The Hub framework has demonstrated that it is possible to realise complex agreements involving multiple academic and industry parties, that would otherwise be challenging to achieve in the absence of such a supporting legal structure.
Unlocking the innovation potential of research infrastructures
Multi-stakeholder collaboration, such as academia-industry partnerships, is one of the cornerstones of successful translational research, and therefore a priority for EATRIS’ mission. Working together with a company increases the chances of translating research findings into patient benefit, and several of the Hub’s projects have received follow-on funding based on promising results.
The five EATRIS institutions involved in the Hub benefit from gaining access to expertise and resources of a global top 10 pharma company, providing a unique setting for patient-centric, academically driven research complementing industry’s own strengths. A public-private collaboration brings new networking and provides access to translational resources that are frequently (still) very rarely accessible to academic researchers.
From the operational perspective, a clear legal and governance structure added much value to the successful start by all partners. A single point of contact offered by EATRIS ERIC provides capacity for overall coordination, legal and operational support, as well as an independent negotiator when needed. Anton Ussi, EATRIS Operations & Finance Director said: “The Innovation Hub is a fantastic example of how a European research infrastructure can fulfil its role as enabler of research and accelerator of innovation. We are now looking at setting up more Innovation Hubs in Europe and globally, so we can continue translating research findings into benefit for patients.”
Read more about the renewal of the Innovation Hub collaboration here.
















