View and download the EATRIS 2023-2026 Strategic Plan as an interactive PDF here.
Thanks to the coordinated efforts of our community, EATRIS now occupies a central position in the European translational medicine infrastructure domain and are positioned as a key driving force for delivering personalised and precision medicine for the benefit of European citizens.
The 2023-2026 EATRIS Strategic Plan launches today, along with a new tagline for the organisation: “Science beyond barriers. Medicine beyond borders.“. As outlined in the document, we plan to exploit the capacities that are available within our infrastructure and beyond, synchronizing the activities of multiple scientific disciplines, and different types of actors. The plan aims at nurturing close cooperation between a broad range of different disciplines and sectors, focusing our community efforts on health outcomes for a healthy society.
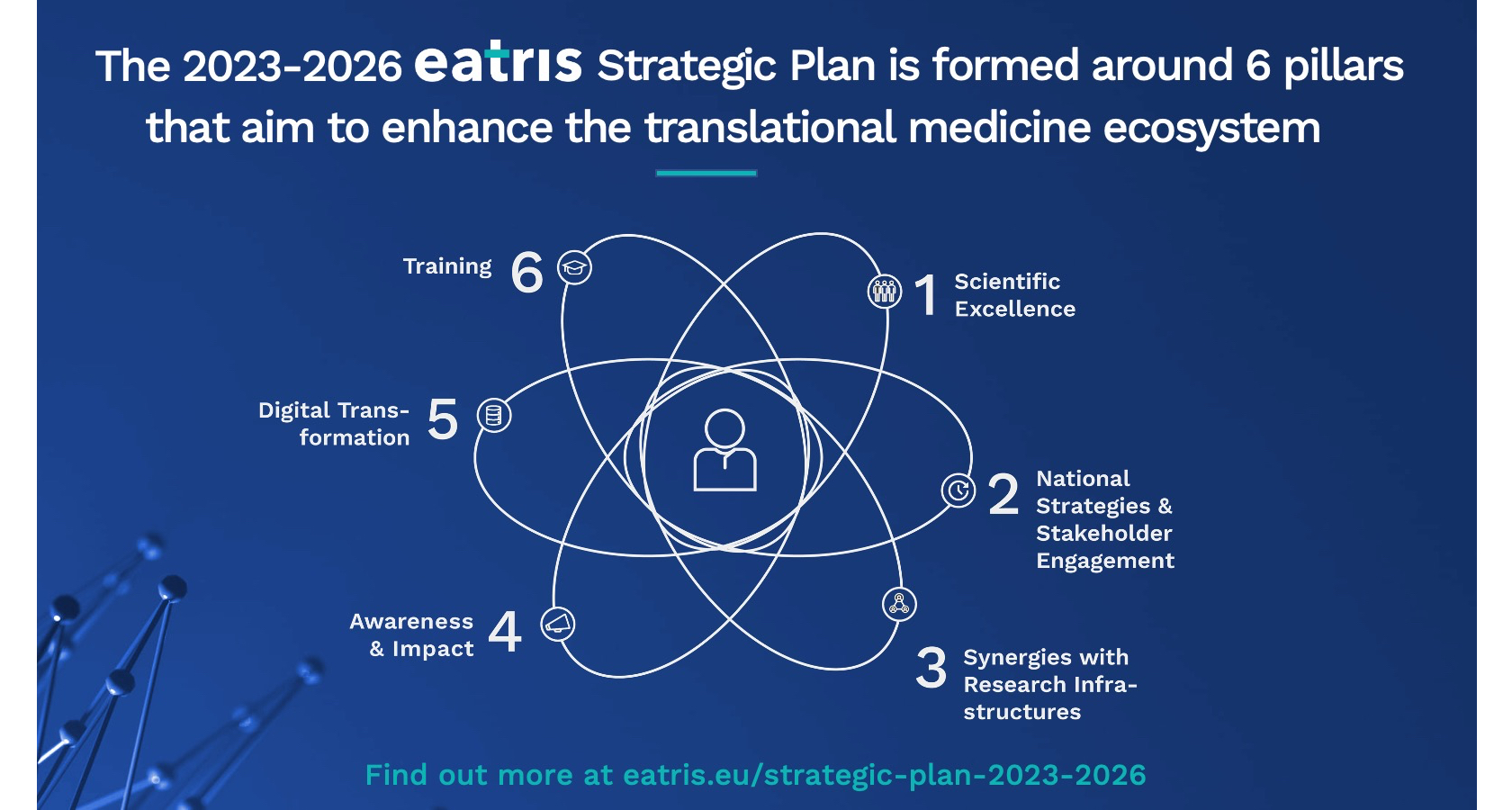
The plan is structured around six pillars that aim to enhance the translational medicine ecosystem. Here are more details on each pillar:
Pillar 1: Exploit our capacities to address unmet medical needs
- Exploit EATRIS Product Platform capacities to develop novel tools for translational medicine
- Activate communities of practice to advance the state of the art
- Nurture our research culture built on quality and reproducibility
- Support academia with biomedical innovation ‘consultancy’ services for the translation of novel interventions that improve human health (late stage of the translational pipeline)
Pillar 2: Engage Member States and other key stakeholders for the advancement of Translational Medicine
- Reinforce cohesion and alignment with EATRIS Member States’ strategies
- Nurture our relationships with governments, competent authorities, funding bodies and policymakers to improve conditions for translation
- Make meaningful patient engagement in translational research the new normal
- Cultivate and extend our global alliances to maximise their transformative impact
- Optimise our role as a bridge between academia and industry for more effective innovation
Pillar 3: Develop synergies within the Research Infrastructure Landscape
- Accelerate the development of the European Alliance of Medical Research Infrastructures (EU-AMRI)
- Bridge capacities and foster synergies with Life Science Research Infrastructures
- Explore interconnections with Research Infrastructures beyond life sciences
- Encourage inter-Research Infrastructures collaborations at a national level
Pillar 4: Sustain and expand awareness of EATRIS
- Nurture and grow EATRIS audiences to consolidate EATRIS positioning as a landmark Research Infrastructure
- Empower EATRIS members as ambassadors of Translational Medicine
- Communicate EATRIS’ impact on human health to support Member States with public awareness
Pillar 5: Accelerate the digital transformation of Translational Medicine
- Define and map the data needs of translational medicine researchers at European scale
- Provide a suite of data services for our users available at the EATRIS Nodes
- Integrate EATRIS data services with key European health data focussed initiatives
- Drive the use of Artificial Intelligence in the Translational Medicine ecosystem through EATRIS data services
Pillar 6: Empower the translational community through training
- Train different audiences to enhance the effectiveness of translating biomedical discoveries into patient benefit
- Build a community of training professionals to enable growth towards a key training organisation for Translational Medicine
Development of the plan
Our Strategic Plan was approved by the EATRIS Board of Governors (BoG) in November 2022 in Amsterdam. It was developed after a round of interviews with our internal community and users in early 2022 and adjusted after an open consultation launched in Q4 of 2022. A total of 51 responses were received and analysed.
- March 2022: Review SP 2019 – 2022 with SWOT analysis performed. Initial outline SP 2023 – 2026 available.
- May 2022: Interviews with internal community achieved. SP 2023 – 2026 outline adjusted and approved by EATRIS Board of Govenors.
- Sep 2022: Full draft SP 2023 – 2026 available. Launch of the open consultation.
- Oct 2022: Closing of the open consultation.
- Nov 2022: Approval SP 2023 – 2026 by EATRIS Board of Govenors.
- Jan 2023: Launch of the Strategic Plan.
Toni Andreu, EATRIS Scientific Director, commented: “Our 2023–2026 Strategic Plan positions EATRIS as a unique contributor for the development of Translational Medicine in Europe, providing fast access to scientific and technological services and developing new tools needed for providing the best healthcare and making science the pillar of a healthier society where every person counts.”

















