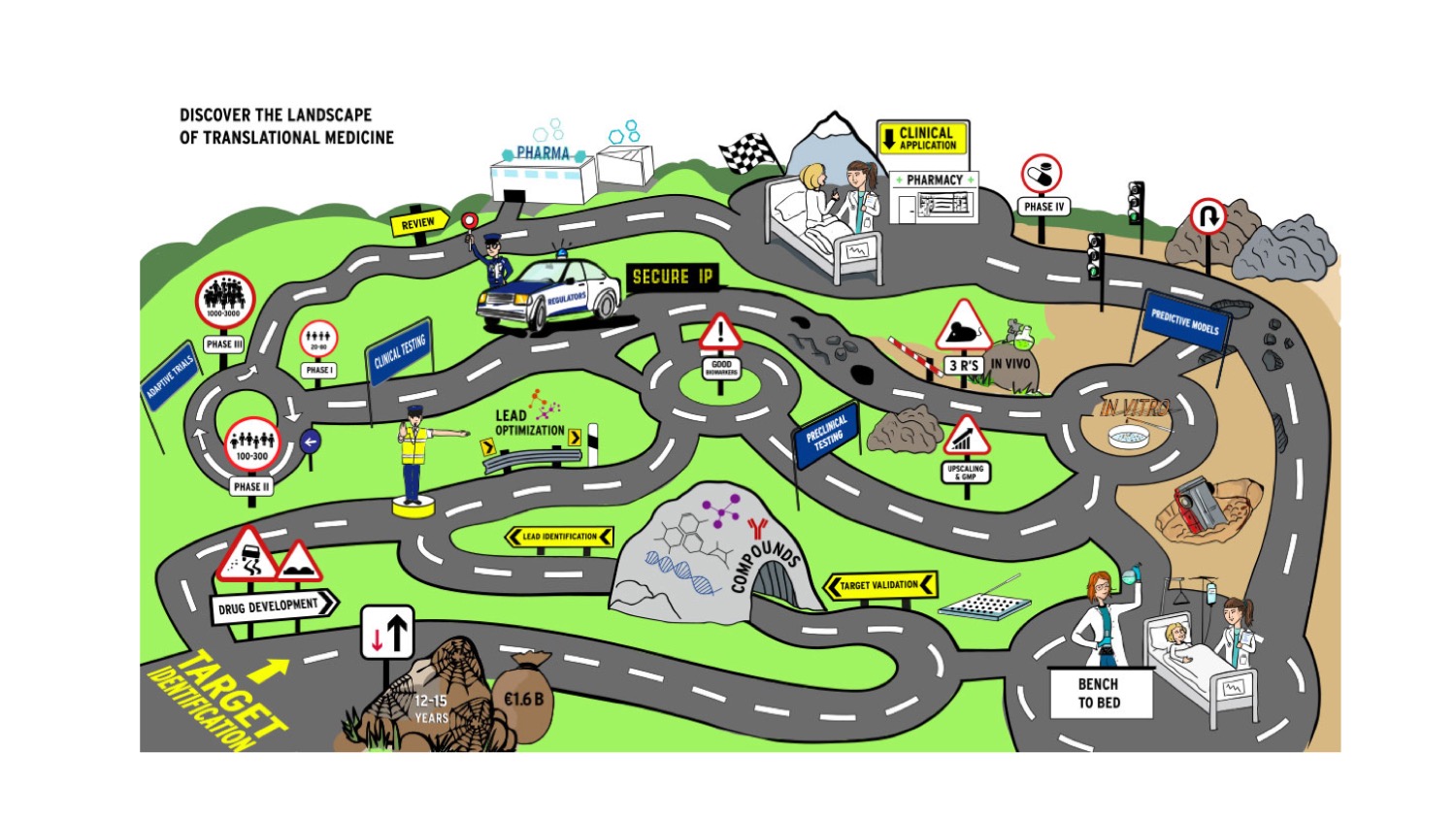
Interested in discovering the landscape of Translational Medicine?
Want to learn about the job profiles of scientists in Industry?
Then join the 2018 course “Translational Research and Medicine Development” which will be hosted by Bayer in Berlin from 16.-20. April 2018 as part of the Marie Curie project ENLIGHT-TEN. The fee of 300 Euro includes the 5 day course in Berlin, catering during the course, two dinners and the e-learning. For more information see here
Registration
First come, first serve. There are only 17 seats for non ENLIGHT-TEN participants, so we recommend applying early. Please submit your application documents (CV, motivation letter, abstract) HERE which will be reviewed by a selection committee. The registration will close on 15 January 2018.
EXPERIENCE
The ENLIGHT-TEN summer school 2018 builds on the experience of the C-COMEND courses on translational research and medicine development which in 2016 & 2017 delivered two highly valued courses. This is what the students said:
“It was great to get such a detailed overview of translational medicine from all those different perspectives. For me meeting a patient and hearing stories form patient’s families were the best part of the course.” – Lea-Isabell Schwarze
“If you have the opportunity to take part in this course – do it. It will fundamentally change your view on how your work as a research scientist can make a difference in the real world outside your lab.” – Konstantin Kuhne
“Very interactive course with a non-traditional approach. The course reflects the reality of translational medicine: getting a lot of people with different backgrounds to work together as a team.” – Miriam Ayuso
“My expectations to the course were very high, and they were fulfilled.” – Sandra Heckers
“It was a difficult decision to leave the lab for 5 days but it absolutely worth it.” – Kevin Ferreira
CONFIRMED SPEAKERS
- Bob Harris, Professor Immunotherapy, Karolinska
- Matthias Gottwald, Head of R&D Policy & Networking, Bayer AG
- Keith Williams, Director, KW Drug Developments
- Jörg Lippert Global Head Clinical Pharmacometrics, VP at Bayer AG
UPON SUCCESSFUL COMPLETION OF THE COURSE PARTICIPANTS WILL…
- have gained a holistic understanding of the medicines development process
- have met and discussed with experts from academia and pharma at eye level
- have heard and discussed real-world stories presented by those who were involved
- have learned about newest approaches in the medicines development sciences
- have networked with peers from different research areas and different countries
FIND OUT MORE ABOUT
C-COMMEND : www.eatris.eu/c-comend.html
ENLIGHT-TEN: www.enlight-ten.eu
Try our interactive roadmap of translational medicine
The course is part of the ENLIGHT-TEN project which has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No.: 675395.