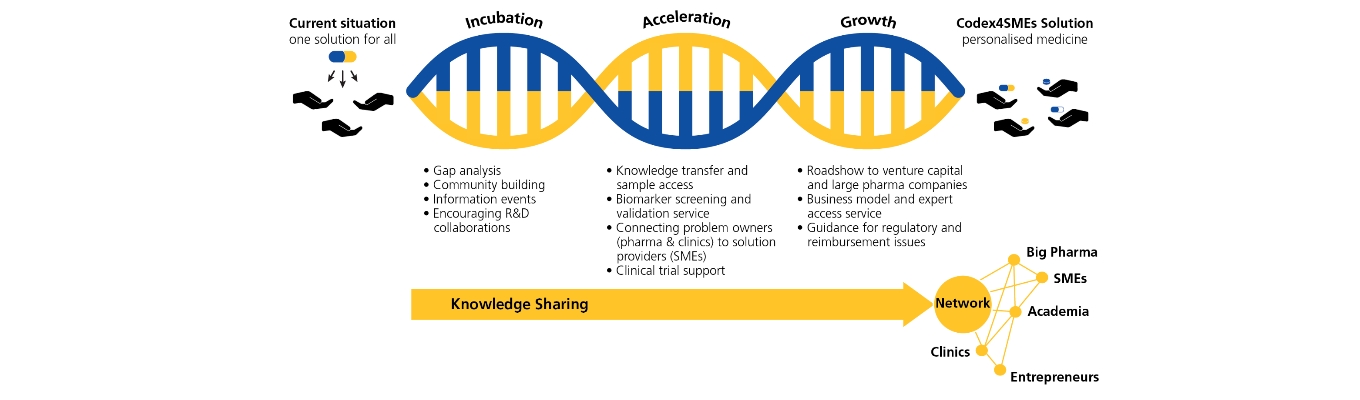
The Interreg project Codex4SMEs (Companion diagnostics expedited for small and medium-sized enterprises) has established a transnational network to support SMEs in their companion diagnostic (CDx) development.
With the goal to improve healthcare by enhanced adoption of personalised medicine in North-West Europe, 11 partners from seven countries have combined their capacities in order to expedite CDx development by SMEs.
A broad offering of services is available to SMEs to improve their innovative capabilities via support schemes available via the Interreg website. The efficacy and efficiency of Personalized Medicine are heavily dependent on the understanding of the cause of disease on the molecular level. CDx are a tool that can assist in the mapping of the disease mechanisms and improve the outcome of precision treatments.
However, the development of such tools has so far been highly time-consuming and costly. As a result, they are presently used only in the context of very few treatments despite their potential wide applicability.
Offering for SMEs:
- Sample access service
- Biomarker validation
- Knowledge transfer service of biomarker/bio-banking
- Access to an ecosystem of 11 partners
- Business model and expert access service for business growth and scaling
- Tailored support dependent on the product TRL levels of the SMEs
You can find more information on the open call website.