| Technology platform | Vaccines |
|---|
TRANSVAC: European Training in Vaccinology
The fourth call is open from 1 April 2019 – 1st June 2019. It covers 3 modules:
- M6 – CyTOF (Cytometry by time‐of‐flight) conducted at CEA, Fontenay-aux-Roses, France (19-20 September 2019)
- M7 – Flow Cytometry organized also by CEA, Fontenay-aux-Roses, France (18-19 September 2019)
- M9 – Key considerations and best practices for viral vaccine process development, scale-up and implementation at manufacturing scale including single use technologies, organized by VFI together with Merck at Merck/M Lab premises in Molsheim, France (10-11 September 2019)
Participants will be selected by a course selection panel.
The course is offered free of charge. However selected applicants will have to cover their own travel and visa expenses and accommodation (excluding M9, where accommodation will be provided by organizers).
Candidates working in a European Union Member State or Associated Member State as well as candidates from other countries who are working in vaccine development projects funded by the European Commission (EC) or European and Developing Countries Clinical trial Partnership (EDCTP) are eligible to apply. Other candidates can also apply, but priority will be given to the groups mentioned above.
INTERESTED IN THIS COURSE?
Fill out the application form and submit before 1st June 2019 to transvacinfo@euvaccine.eu. You will receive a notification of the application outcome by 1st July 2019.
For more information about the training modules offered by TRANSVAC, the eligibility criteria and the application process, please visit here.
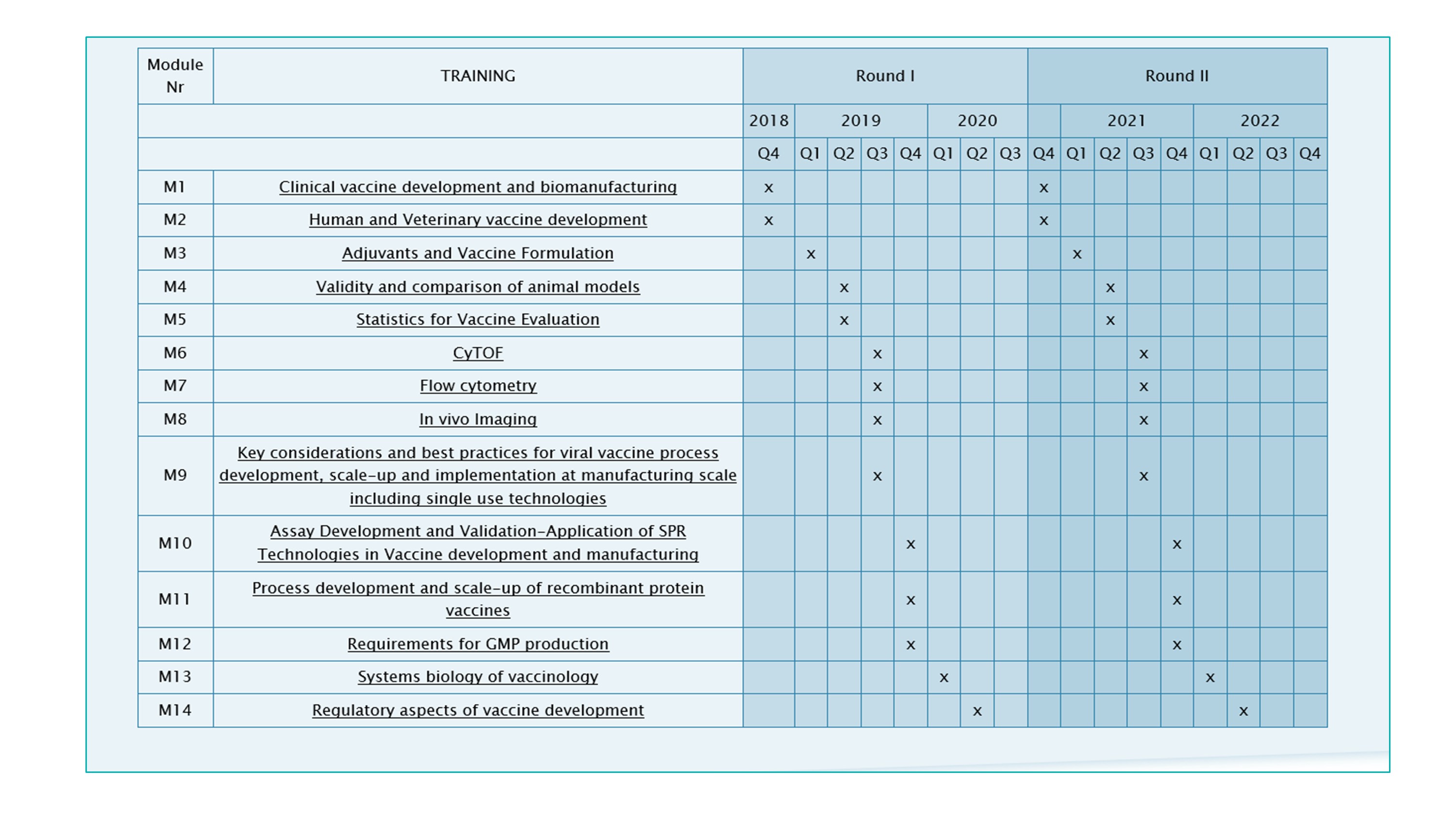
TRANSVAC2 TRAINING MODULES
The training modules are part of a series of 14 training modules dedicated to vaccinology set up by the TRANSVAC2 consortium at leading European centres. The modules can be combined to create customised international courses on vaccine R&D. Two rounds of customised training courses are planned. Participants can select topics as needed in their field of vaccine development, and the timelines of the various modules will be harmonised in a way that allows a logical continuation from one topic to the other.
For more information please check the dedicated TRANSVAC training webpage here or contact the TRANSVAC team (transvacinfo@euvaccine.eu).