| Date & Time | 28-29 March 2023 |
|---|---|
| Location | Amsterdam, the Netherlands |
Training scientists in vaccine research and development is crucial in order to sustain Europe’s excellence in this field. The TRANSVAC2 Consortium has set up training modules at leading European centres (in collaboration with WP18 partners) that can be combined to create customised international courses on vaccine research and development (R&D). Participants can select topics as needed in their field of vaccine development, and the timelines of the various modules will be harmonised in a way that allows a logical continuation from one topic to the other.
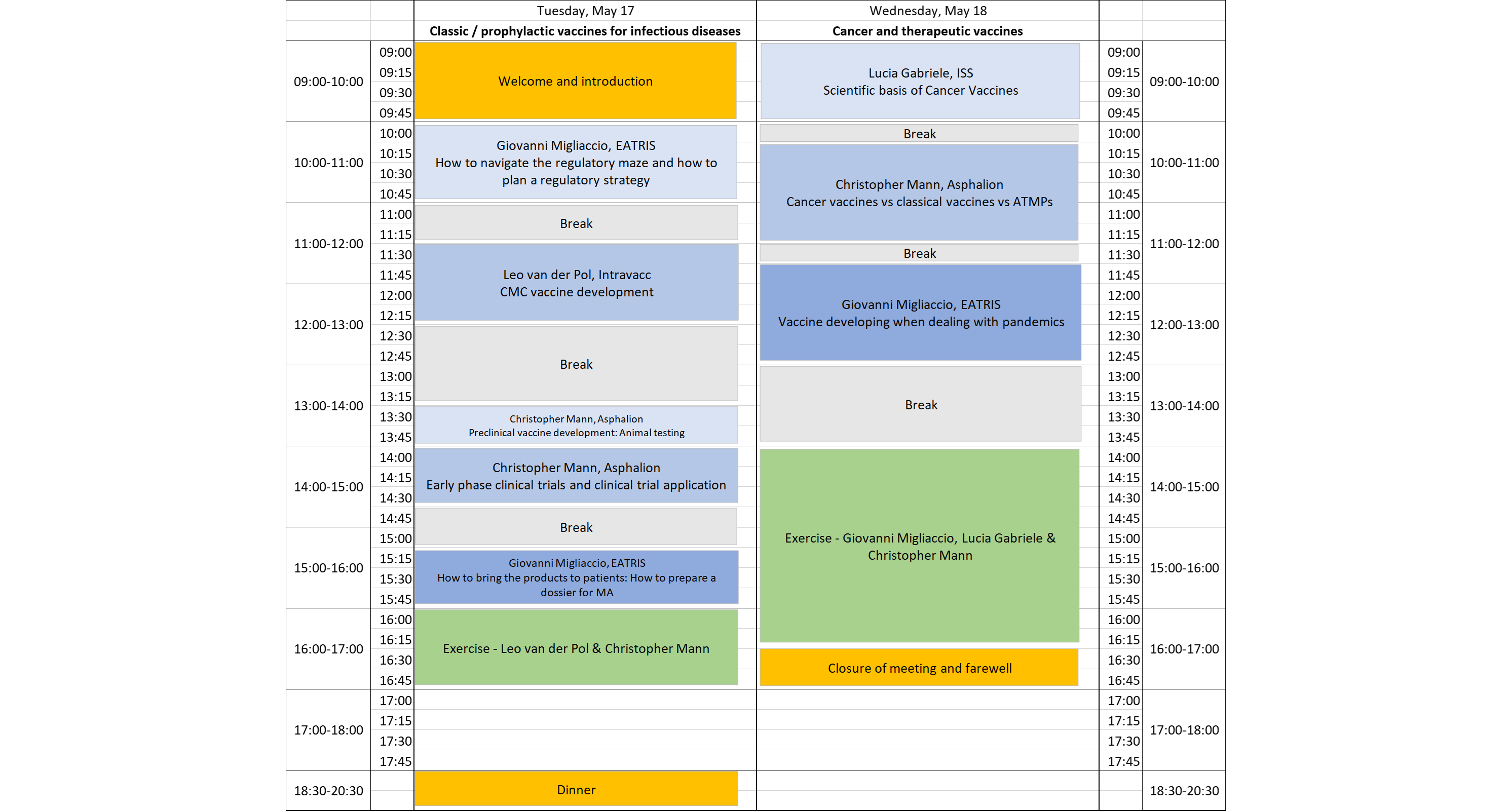
Organised by EATRIS, this two-day training workshop on vaccine development will introduce participants to planning a regulatory strategy for vaccines with an emphasis on the early phases of development.
Day 1 will focus on prophylactic vaccines, including lectures and case studies on how to navigate the regulatory maze and how to plan a regulatory strategy, preclinical and Phase I-II of vaccine development, how to prepare a dossier for Medicine Agencies, CMC development.
Day 2 will focus on cancer vaccines, with an introduction to the scientific part to understand the regulatory part better.
The course is designed for professionals working on vaccine development who are in need of an introduction to the regulatory issues associated with the field.
Applications are open until 31 December 2022 here.