


|
|
| Address | Rua Pedro Nunes 3030-199 Coimbra Portugal |
|---|---|
| Platforms | Pending |

The Instituto Pedro Nunes (IPN) offers a Regulatory Support Unit that specializes exclusively in the medical devices (MDs) and in vitro diagnostic medical devices (IVDs) sectors. This department provides a range of services designed to facilitate the process of certification of these devices while taking into account global regulations from several markets, including Europe, the United States of America, Brazil, and the United Kingdom.
Moreover, the Regulatory Support Unit also provides support in clinical trial submission, through preparation or review of documentation, internal audits of the system and/or product, and specialized consulting hours and professional training. By availing themselves of IPN’s Regulatory Support Unit services, clients can streamline their regulatory compliance and market access process, making it easier to focus on product development and innovation.
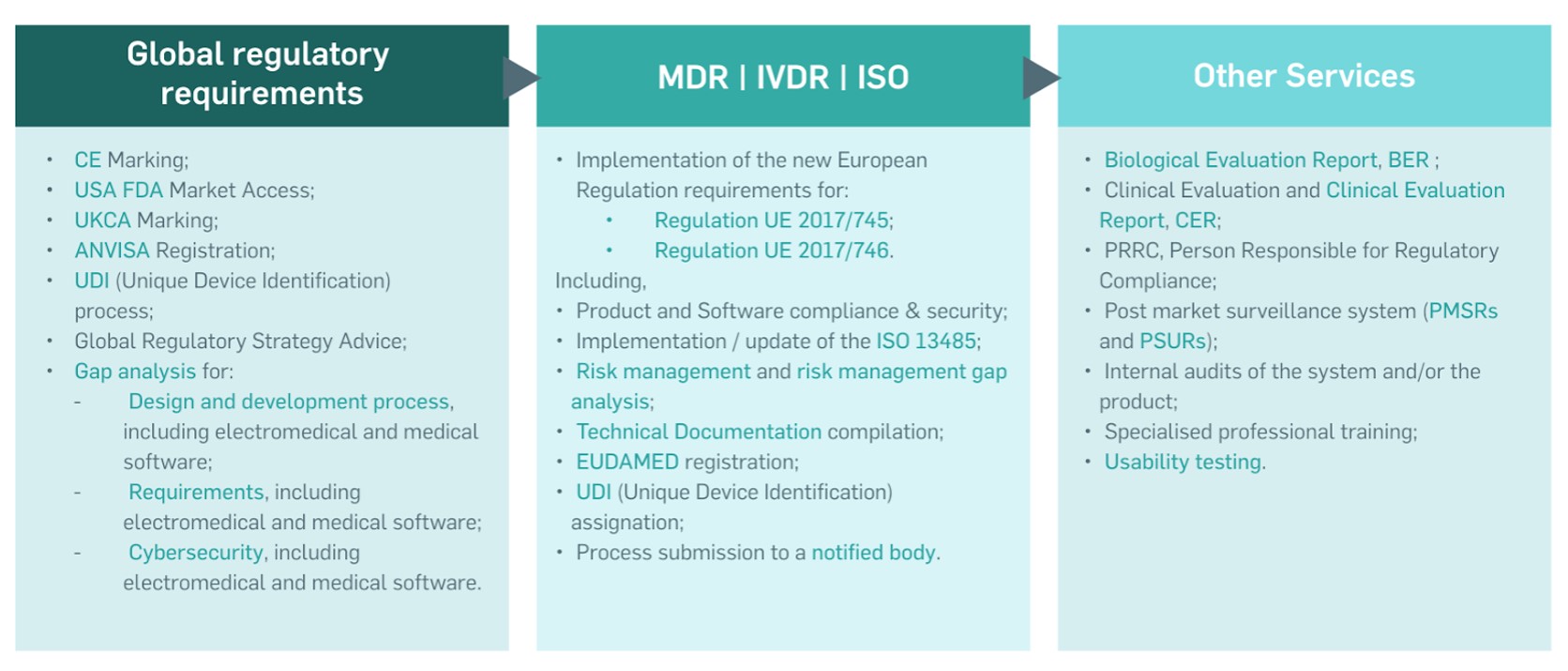
Among others, in the last two years, the Regulatory Support Unit has helped companies implement the requirements of the Medical Devices Regulation (Regulation (EU) 2017/745 – MDR) or in vitro Diagnostic Medical Devices Regulation (Regulation (EU) 2017/746 – IVDR) for more than 50 devices, and CE marking for over 100 devices. Their offer of specialised professional training has helped over 60 companies, with over 150 people trained. IPN prides itself on its ability to provide comprehensive support to its clients and support their medical or in vitro diagnostic devices to successfully reach the market.
The services provided by IPNlas’ Regulatory Support Unit include support in implementing the requirements of the MDR or IVDR, UDI assignation process, EUDAMED database registration, complete CE Marking process, ANVISA process (Brazil), USA FDA market access, UK certification process, and QMS implementing/updating according to ISO 13485 and ISO 9001.
Instituto Pedro Nunes (IPN) offers expert Regulatory Support and (Early) Health Technology Assessment services to clients in the medical devices and in vitro diagnostic medical devices sectors.
The IPN through the Laboratory for Automation and Systems (IPNlas) has a Regulatory Support Unit, which is a global consulting unit specialising exclusively in the medical devices (MDs) and in vitro diagnostic medical devices (IVDs) sectors and has expertise in helping clients achieve global compliance in multiple markets. This unit provides a portfolio of services aimed at supporting companies and facilitating the process of certification of their MDs or IVDs taking into account global regulations, from several markets (European Union, Brazil, USA and UK).
Services provided by IPNlas’s Regulatory Support Unit include:

(Early) Health Technology Assessment services:
In addition to Regulatory Support services, IPNlas also provides (Early) Health Technology Assessment services that focus on providing solutions developers with the necessary tools and information to create value for their solutions and accelerate ICT solutions adoption in healthcare systems. The framework is built on four value pillars, namely: Appropriate care to achieve patients’ goals, achievement of the best possible outcomes with available resources, equitable resource distribution across all patient groups, and maximizing health outcomes achieved per euro spent. Services provided include proof of concept evaluation, early usability and safety evaluation, early clinical suitability, and early economic project sustainability.

















