| Project Acronym | ISIDORe |
|---|---|
| Funding Programme | Horizon Europe |
| Budget | 20,998,624.00 |
| Coordinator | ERINHA |
| Website | https://isidore-project.eu/ |
| Starting-ending date: | 1 February 2022 - 1 January 31 2025 |
The project has now ended.
About ISIDORE
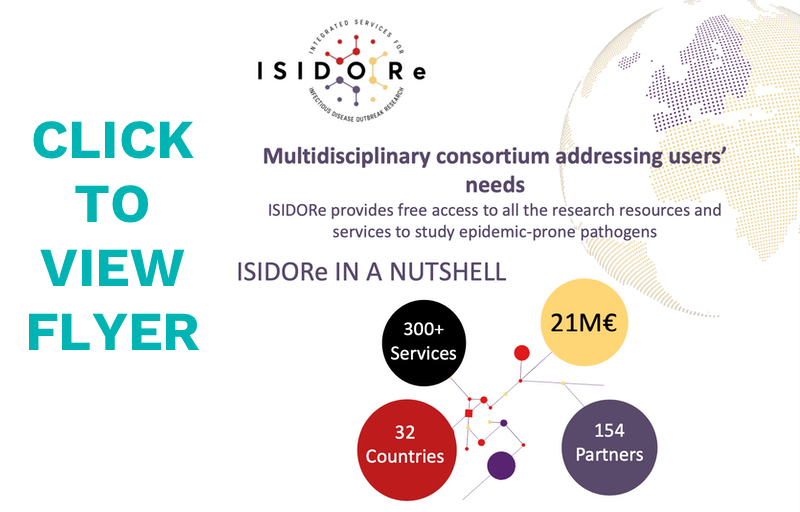
The ISIDORe consortium will improve Europe’s global service and research capacities by becoming a key, ERA-embedded instrument for supporting research on epidemic-prone pathogens, and aims at contributing to fighting the rise of the SARS-CoV-2 variants through a global, integrated and challenge-driven approach by providing fast access to cutting-edge resources and services to scientific user communities for supporting the evidence-based development or adaptation of countermeasures in times of emergency. Secondly, the project aims at contributing to Europe’s readiness to any epidemic-prone pathogen through a global, integrated and preparedness-driven approach by providing access to cutting-edge resources and services to scientific user communities for supporting their research projects in the field of infectious diseases in “peaceful times” as well as during epidemics.
Submit an ISIDORe-EATRIS TNA proposal
If you wish to submit a TNA research proposal or regulatory services, please download this ISIDORe EATRIS TNA Application form, complete it, sign it and email it to Patricia Carvajal (patriciacarvajal@eatris.eu) and David Morrow (davidmorrow@eatris.eu).
ISIDORE Consortium
ERINHA, other ESFRI Life Sciences research infrastructures and networks, all conducting activities that are highly relevant to the Research infrastructure services for rapid research responses to COVID-19 and other infectious disease epidemics topic, formed a multidisciplinary consortium to respond to the new research needs that constantly arise from the emergence of SARS-CoV-2 variants.
It has mobilised and engaged over 150 access providers from 31 different countries that committed to dedicate part of their capacities to the ISIDORe project to offer scientific communities access to their facilities, resources and equipment, and ultimately advance research on SARS-CoV-2 variants and infectious agents of public health concern.
The development of the ISIDORe project, and the capacities presented in the proposal, are the result of the work and commitment of a ‘core consortium’ of 17 entities:
- 6 EU-funded networks: EVA, VetBioNet, INFRAVEC, Sonar-Global, TRANSVAC, MIRRI;
- 1 nationally-funded surveillance network: EMERGEN;
- 10 legally established ESFRI infrastructures: INSTRUCT, EuBi, EU-OS, ECRIN, EATRIS, BBMRI, INFRAFRONTIER, EMBRC, ELIXIR, and ERINHA.
EATRIS role in the project
EATRIS is the leader of WP12, which involves coordinating the access of selected users to their immune monitoring and profiling services. EATRIS will also provide expertise on regulatory requirements for trial authorisation, ethics committees, fast track procedures, coordinated authorisation in WP15 as co-leader. In close collaboration with ECRIN and TRANSVAC for vaccine trials, EATRIS will focus on the quality of the data, the robustness of the trial and the cost containment.
Concluded TNA projects and key achievements
Below are examples of Transnational Access (TNA) projects in ISIDORe, where EATRIS contributed its services to accelerate the development of new products and protocols for patient benefit. Click on each title and read more about the TNA project.