| Technology platform | Imaging & Tracing |
|---|
EATRIS and GlaxoSmithKline to Extend Translational Molecular Imaging Hub Collaboration with Maturing Project Portfolio
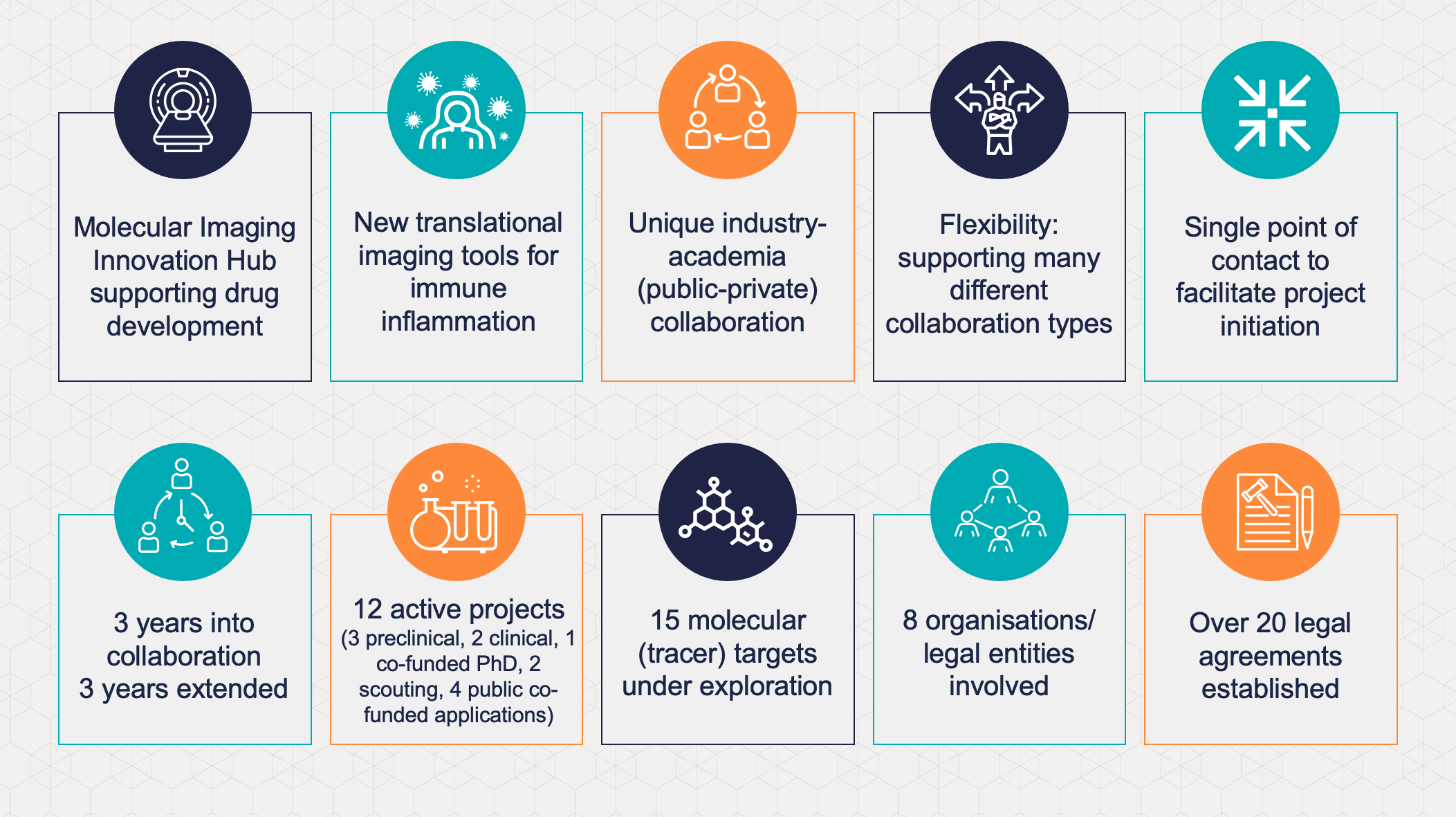
In 2018, EATRIS and GlaxoSmithKline (GSK) announced the establishment of a novel international multi-site collaboration. This collaboration is aimed at the development and implementation of new clinical imaging tools for immune inflammation in a new collaboration format. Now, at the end of the initial period, the EATRIS institutions, in tight collaboration with GSK will continue their collaboration for another 3 years.
Scope
The technical scope of the Hub collaboration covers molecular imaging (SPECT, PET, optical), functional imaging (DCE, DW-MRI) and structural imaging (MRI, US, CT) to support drug development. Finding innovative endpoints for information rich Ph1b studies, performing small clinical and pre-clinical studies in specialised centres is a key topic. Although the aim is to deliver disease agnostic tools that can be validated (pre-)competitively as broadly as possible, current indications of interest include IBD, RA , liver disease, lung and kidney fibrosis, asthma and COPD and pain (neuroinflammation), with oncology as a rapidly emerging area of interest. The use of imaging should provide a deep molecular understanding of the underlying disease pathophysiology, such as inflammation, fibrosis, epithelial barrier integrity and function, and specific cell populations involved (T-cells, macrophages, NK cells, neutrophils and/or myofibroblasts) through the development and/or generation of novel imaging tools..
Current status
During the initial period, the Imaging Hub built a mature project portfolio with 3 currently active preclinical projects, 2 clinical projects, 1 co-funded PhD project, 2 active scouting projects and 4 public co-funding applications submitted. There are currently >15 molecular (tracer) targets on the radar with concepts in various stages of development. The exchange of knowledge and the sharing of tools leads to cross fertilisation and propagates ideas, that greatly enhance the value of this collaboration
Added value for GSK
The GSK clinical imaging and bioimaging teams, working closely with biomarker groups aim to support all Ph1–3 clinical trials utilising imaging. This capacity is supplemented with clinical expertise, knowledge, and infrastructure through an external network, which is strengthened through the Hub academic alliance structure. The flexibility of the framework supports several project types with pre-set terms (IP, publication), ranging from bespoke imaging probe development to investigator sponsored trials, with EATRIS coordinating as a single point of contact. This structure allows versatile arrangements and decisions to be initiated or extended on a case by case basis. Over 20 agreements have been prepared to date (including multilateral CDAs, MTAs, 3rd party licensing, project agreements, external consortium agreements) and proposals can be individually tailored to support the collaboration. The Hub framework has shown that it is possible to realise complex agreements involving multiple external partners, that would otherwise be challenging to achieve in the absence of such a supporting structure; and is successfully contributing to the development and application of innovative imaging methods for inflammatory diseases.
Added value for the Academic Medical Research Institutions
The public-private partnership brings a new dimension for the academic researcher. It gains access to expertise and resources of a global top 10 pharmaceutical company, providing a unique setting for patient centric, academically driven research with complementary strengths. More importantly, new research tools become accessible and can be developed towards a more advanced technological state, in a shared and focused manner. The Hub framework provides a structure where projects are designed to maximise successful delivery of tangible results with industry due diligence to ensure compliance, quality and speed. A project development checklist has been developed as a tool to support this process. Working together with a company increases the chances of translating research findings, and several of the projects have received follow-on funding based on positive results. Similarly, collaborating in areas of common interest has also expanded into co-funding opportunities that are oftentimes underscored with several Letters of Support and Letters of Intent supporting external grant applications.
Outlook
With the first molecular tracers moving towards first–in–human studies, the aim is to validate these disease agnostic translational imaging tools for a broad panel of indications. Subsequently, the project portfolio will need to show proof of how these non-invasive imaging methods can positively impact drug development and/or have added value in a clinical (research) context. The Hub Steering Committee has agreed in March 2020 to extend the initial period by another 3–years, resulting in renewal of the collaboration agreement in July 2020 and consolidating early lessons learned.
Over time, and as the Hub partners revise and restructure their own organizations and priorities, the Hub’s flexibility can (continue to) adapt and accommodate these changes. Moreover, the possibility to expand the Hub concept is actively being explored, either by including an additional funder/collaborator, or by creating a similar initiative with a different scope, leveraging the broad consortium of EATRIS translational research centres.
Quotes from partners of the GSK Imaging Hub Collaboration
Dr Rogier Thurlings, Rheumatologist, Radboudumc, Nijmegen, The Netherlands:
“We are very positive about the collaboration in the GSK Immune Inflammation Imaging Hub. The Hub forms a unique platform for interaction between academia and industry. Its dedication towards clinical implementation increases the chance that our patients profit from imaging guided drug development.”
Anton Ussi, Operations and Finance Director of EATRIS:
“The renewal of the Hub collaboration involving GSK and six academic institutions of our consortium shows the added value of EATRIS in the creation and execution of a unique and tailored public private partnership. We are very excited about the growing portfolio of this novel collaboration format and are looking forward to seeing the impact of its emerging molecular imaging tools in experimental medicine and drug development. The EATRIS central support office is dedicated to the construction of bespoke international research collaborations from concept to execution of contracts.“
















